(Isstories Editorial):- Dongguan, Guangdong Sheng Nov 19, 2025 (Issuewire.com) – In the evolving landscape of global footcare and orthopedic footwear manufacturing, material innovation plays a decisive role in determining comfort, performance, and product longevity. For distributors, brand owners, and manufacturers, finding the right partner for orthopedic shoe material bulk purchase is essential to maintaining product quality and cost efficiency. Among the most trusted and innovative suppliers in this field, Dongguan Suscong Healthcare Co., Ltd. stands out as a global leader in advanced footcare solutions and insole materials — offering high-performance TPU, EVA, and gel components designed for orthopedic, sports, and medical footwear.
The Global Footcare and Orthopedic Footwear Market: A Growing Industry
The orthopedic footwear and footcare industry has witnessed exponential growth in recent years, fueled by an aging global population, increased health awareness, and the booming demand for customized comfort products. Consumers are no longer satisfied with standard insoles; they now expect scientifically engineered designs that support posture correction, relieve pressure, and improve long-term mobility.
More on Isstories:
- Immigrant Entrepreneurship in America by Gorm Tuxen Achieves #1 Amazon Bestseller Status
- Colle AI Formalizes Strategy Around Cross-Chain Asset Versioning Infrastructure.
- Imagen Network Sets Forth Multi-Perspective Rendering Initiative for Immersive Asset Creation
- Apolosign Digital Wall Calendar Tackles “Proxy Parenting” in U.S. Homes
- 25 Countries for Solo Travelers to Discover Themselves
According to market research reports, the global orthopedic footwear market is expected to reach over USD 8 billion by 2030, with Asia-Pacific emerging as one of the fastest-growing regions. This growth is driven by urbanization, rising disposable incomes, and a growing focus on preventive healthcare. In this context, material quality and innovation have become vital competitive advantages.
Established in 2011, Dongguan Suscong Healthcare Co., Ltd. has been at the forefront of this transformation. From its manufacturing base in Dongguan City — a hub for China’s footwear and healthcare production — Suscong provides over 500 types of footcare products, including insoles, heel cups, cushions, and orthopedic components. The company’s professional R&D team and QC department ensure each product meets strict international standards while supporting custom OEM and ODM projects for leading global brands in over 70 countries.
Founder Jeff Zhang began this journey in 2005 by establishing BDAC Company in Beijing, focusing on distributing high-quality insoles made in his hometown. Recognizing the need for greater control over production quality and service reliability, he built Suscong in Dongguan six years later. Today, the company operates advanced assembly lines and a vertically integrated supply chain to deliver precision-engineered orthopedic materials on a global scale.
Certified Excellence: Building Trust Through Global Standards
In a market where trust, safety, and consistency are paramount, Suscong Healthcare has built a reputation for excellence backed by international certifications. Each certification represents a commitment not only to product quality but also to corporate responsibility, environmental protection, and ethical manufacturing practices.
Core Certifications and Accreditations Include:
FDA (U.S. Food and Drug Administration): Ensures compliance with strict safety and material standards for products used in medical and orthopedic applications.
ISO 13485: Certifies that Suscong meets global requirements for the design and manufacture of medical devices, emphasizing traceability and risk control.
ISO 9001: Recognizes the company’s robust quality management system across R&D, production, and logistics.
BSCI & SMETA Pillar 4: Demonstrates commitment to social responsibility, workplace safety, and ethical labor practices.
WCA (Workplace Conditions Assessment): Confirms a healthy, compliant, and fair working environment.
DUNS Registered: Establishes credibility for global trade and supplier verification.
ENViSA & CE: Ensures conformity with European environmental and product safety directives.
GRS (Global Recycled Standard): Highlights sustainable sourcing and use of eco-friendly materials in production.
FSC (Forest Stewardship Council): Verifies that raw materials are sourced responsibly from sustainable suppliers.
MHRA (UK), KFDA (Korea), and SFDA (China): Approvals from international health authorities allowing global distribution of orthopedic and medical-grade products.
Through these accreditations, Dongguan Suscong Healthcare Co., Ltd. demonstrates its unwavering dedication to producing safe, sustainable, and high-quality materials for global footwear and orthotic brands.
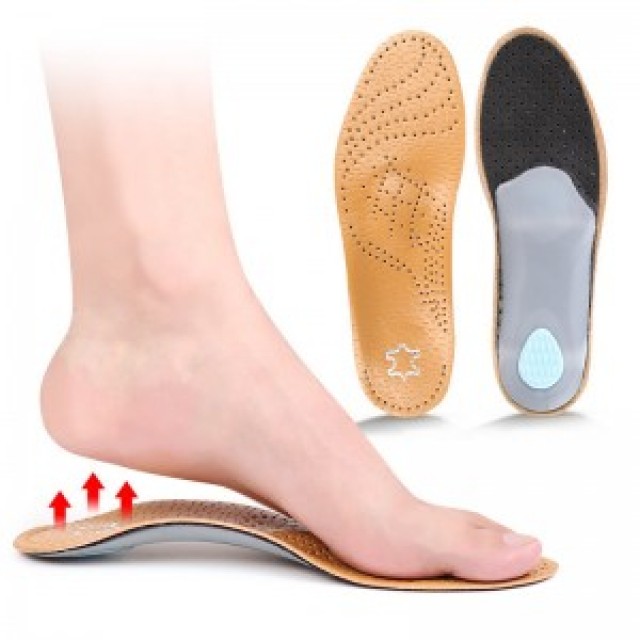
Material Comparison: TPU, EVA, and Gel Components in Orthopedic Shoe Production
When purchasing orthopedic shoe materials in bulk, choosing between TPU (Thermoplastic Polyurethane), EVA (Ethylene Vinyl Acetate), and Gel components depends on performance needs, target markets, and budget considerations. Each material offers unique benefits that make it suitable for specific product types.
1. TPU (Thermoplastic Polyurethane): Precision and Durability
TPU is one of the most advanced materials in the orthopedic and footwear industries. Known for its elasticity, resilience, and high tear resistance, TPU is perfect for performance-driven applications.
Advantages:
- Exceptional strength and flexibilityunder repeated stress.
- Excellent resistance to wear, oil, and chemicals.
- Allows for precision molding, supporting complex ergonomic designs.
- Delivers superior energy return for athletic or corrective footwear.
Limitations: Slightly heavier and costlier compared to EVA.
Ideal For: Sports insoles, orthopedic corrections, and high-impact comfort systems.
2. EVA (Ethylene Vinyl Acetate): Lightweight and Affordable Cushioning
EVA remains a classic material for insoles due to its light weight, affordability, and excellent shock absorption. It is ideal for brands seeking balance between comfort and cost-efficiency.
Advantages:
- Soft, comfortable feel for long-term wear.
- Low density makes shoes lighter and more flexible.
- Easily customizable for color, thickness, and hardness.
- Heat moldable, suitable for orthotic shaping.
Limitations: Compresses over time, reducing longevity.
Ideal For: Casual shoes, children’s footwear, and general orthopedic products.
3. Gel Components: Advanced Comfort and Medical Relief
Gel materials are increasingly favored for premium orthopedic insoles and medical footwear, offering adaptive support and superior cushioning.
Advantages:
- Exceptional shock absorption, reducing joint stress.
- Provides targeted pressure relief, ideal for diabetic or post-surgery footwear.
- Enhances comfort and stability in sensitive-foot conditions.
Limitations: Higher material cost and added product weight.
Ideal For: Medical-grade orthotics, therapeutic insoles, and recovery footwear.
By combining these materials — such as EVA with TPU for structural support or Gel inserts within EVA foam for added cushioning — manufacturers can achieve superior comfort, performance, and longevity in their orthopedic footwear designs.
Why Choose Suscong Healthcare for Orthopedic Material Supply
Dongguan Suscong Healthcare Co., Ltd. goes beyond traditional material supply by providing integrated services that include material selection consultation, custom engineering, and certified production. As one of China’s leading wholesale insole and orthopedic material suppliers, Suscong’s competitive advantages include:
Comprehensive Material Expertise: In-depth knowledge of TPU, EVA, Gel, and hybrid materials for various orthopedic applications.
Professional R&D and QC Teams: Ensuring innovation, quality control, and compliance with international standards.
Strong Supply Chain: Access to top-tier raw material providers for stable pricing and fast delivery.
OEM/ODM Customization: Tailored designs to match each client’s performance, comfort, and branding goals.
Sustainability Commitment: Adherence to GRS and FSC certifications, promoting eco-conscious production.
With over a decade of industry experience, Suscong Healthcare has supported numerous global footwear and medical brands in developing ergonomic, high-quality, and market-ready orthopedic products.
Conclusion: The Future of Orthopedic Footwear Materials
The next era of orthopedic footwear innovation will be defined by the intelligent use of materials — balancing comfort, sustainability, and scientific precision. By mastering the engineering of TPU, EVA, and Gel components, and providing certified, large-scale production capabilities, Dongguan Suscong Healthcare Co., Ltd. continues to empower global partners with solutions that redefine foot health and comfort.
For businesses seeking a reliable, certified, and innovative partner for orthopedic shoe material bulk purchase, Suscong offers the ideal combination of technology, quality, and service.
For partnership inquiries or more information, please visit the official website: https://www.suscong.com/

Dongguan Suscong Healthcare Co., Ltd.
[email protected]
+86-13509235363
https://www.suscong.com/
This article was originally published by IssueWire. Read the original article here.